Jilab Inc. has a modern histopathology laboratory, which is specialized in providing services for academic research and for pharma industry.
Jilab offers histotechnical services, including tissue processing, sectioning, staining, immunohistochemistry, whole slide imaging, quantitative image analysis and reporting. Additionally, Jilab offers RNA extraction and RNAseq services. The laboratory is run by Prof. Jorma Isola, (MD, PhD), who is board-certified pathologist with 30-year experience in histopathology.
Jilab has developed digital quantitative morphometry tools for celiac disease biopsy pathology, including morphometric methods for analyzing duodenal villus atrophy, crypt hyperplasia, and inflammation.
Jilab laboratory is certified by an international quality assurance organisations CLIA and ISO15189. Our IT systems have undergone quality inspection by CyberVadis Inc.
VH/CD and IEL counting using proprietary image analysis software
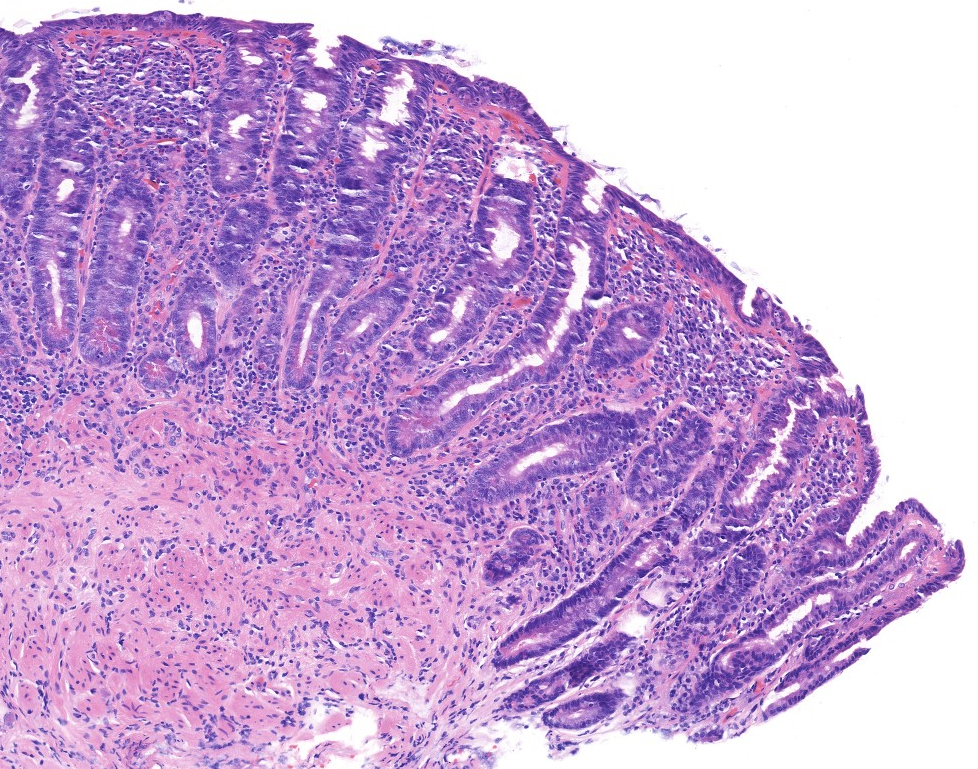
Celiac disease Histology (H&E):
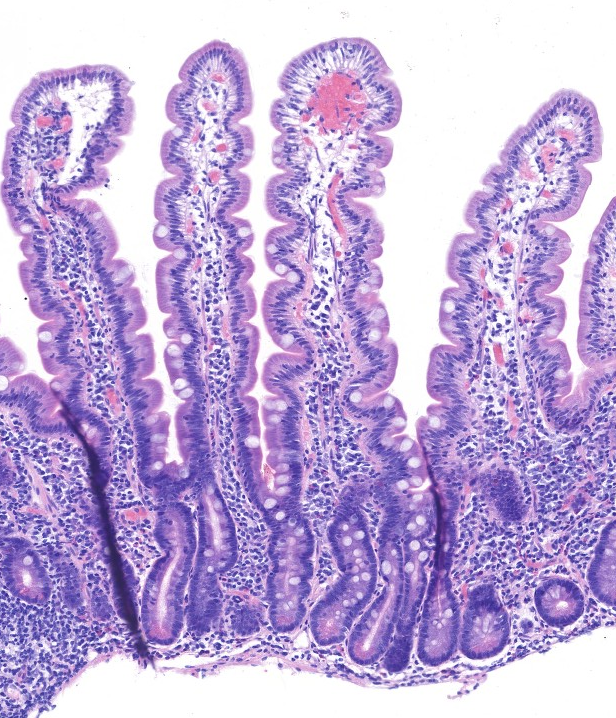
Normal mucosal biopsy Histology (H&E):
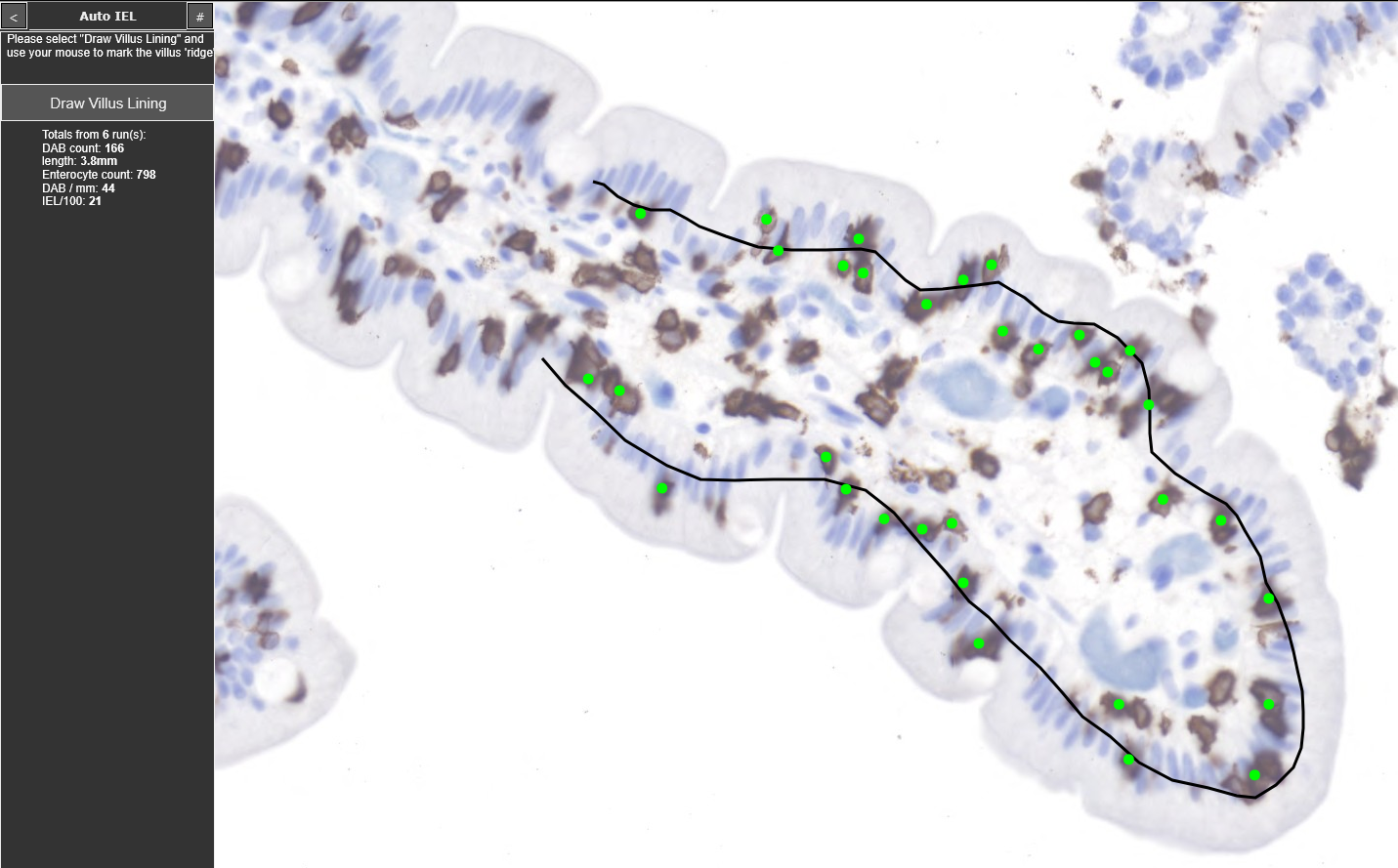
Intraepithelial lymphocyte count on CD3-IHC staining
Villus height : Crypt depth measurement
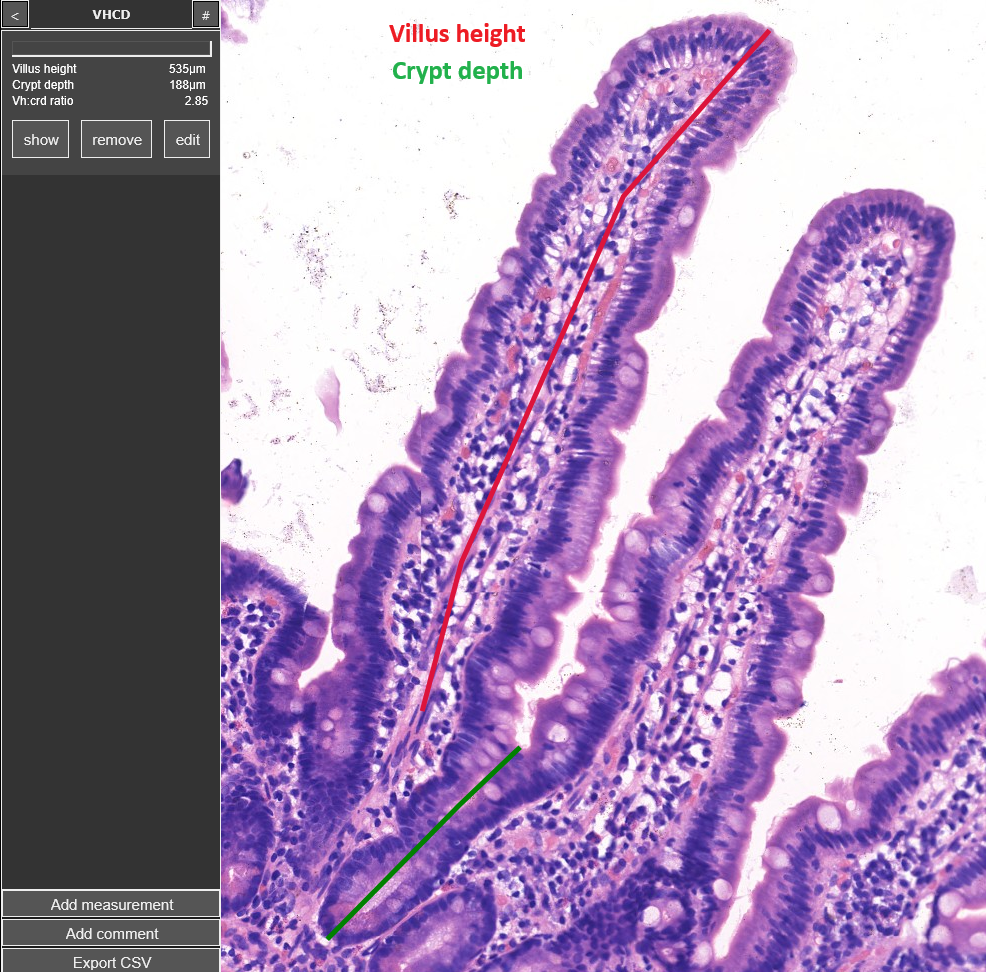
VHCD digital image analysis
IEL digital image analysis
References:
Cellier C et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: a phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol Hepatol 2019 Dec;4(12):960-970. doi: 10.1016/S2468-1253(19)30265-1. Epub 2019 Sep 4.
Daveson A, Popp A, Taavela J, Goldstein K, Isola J, Truitt K, Mäki M, Anderson RP, on behalf of the RESET CeD Study Group. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten‐free diet GastroHep https://doi.org/10.1002/ygh2.380. First published: 17 November 2019
Dotsenko V, Oittinen M, Taavela J, Popp A, Peräaho M, Staff S, Sarin J, Leon F, Isola J, Mäki M, Viiri K. Genome-wide transcriptomic analysis of intestinal mucose in celiac disease patients on a gluten-free diet and post gluten challenge. Cell Mol Gastroenterol Hepatol.Cell Mol Gastroenterol Hepatol. 2021;11(1):13-32. doi: 10.1016/j.jcmgh.2020.07.010. Epub 2020 Jul 31.
Lamacchia C et al. Healthy and pro-inflammatory gut ecology plays a crucial role in the digestion and tolerance of a novel Gluten Friendly™ bread in celiac subjects: a randomized, double blind, placebo control in vivo study. Food & Function 2022 Feb 7;13(3):1299-1315. doi: 10.1039/d1fo00490e.2022
Lähdeaho ML, Scheinin M, Vuotikka P, Taavela J, Popp A, Laukkarinen J, Koffert J, Koivurova OP, Pesu M, Kivelä L, Lovró Z, Keisala J, Isola J, Parnes JR, Leon F, Mäki M. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol Hepatol. 2019 Dec;4(12):948-959. Epub 2019 Sep 4.
Popp A, Taavela J, Anderson B, Isola J, Mäki M. Small intestine digital histomorphometry for celiac disease. Popp A et al. DDW 2016 Congress abstract & poster presentation.
Schuppan D et al. A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease. N Engl J Med. 2021 Jul 1;385(1):35-45. doi: 10.1056/NEJMoa2032441.
Taavela J, Koskinen O, Huhtala H, Lähdeaho ML, Popp A, Laurila K, Collin P, Kaukinen K, Kurppa K, Mäki M. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One. 2013 Oct 11;8(10):e76163.
Taavela J, Viiri K, Popp A, Oittinen M, Dotsenko V, Peräaho M, Staff S, Sarin J, Leon F, Mäki M, Isola J. Histological, immunohistochemical and mRNA gene expression responses in coeliac disease patients challenged with gluten using PAXgene fixed paraffin-embedded duodenal biopsies. BMC Gastroenterol. 2019 Nov 15;19(1):189.
Taavela J et al. Apolipoprotein A4 Defines the Villus-Crypt Border in Duodenal Specimens for Celiac Disease Morphometry. Front Immunol. 2021 Jul 29;12:713854. doi: 10.3389/fimmu.2021.713854. eCollection 2021.
